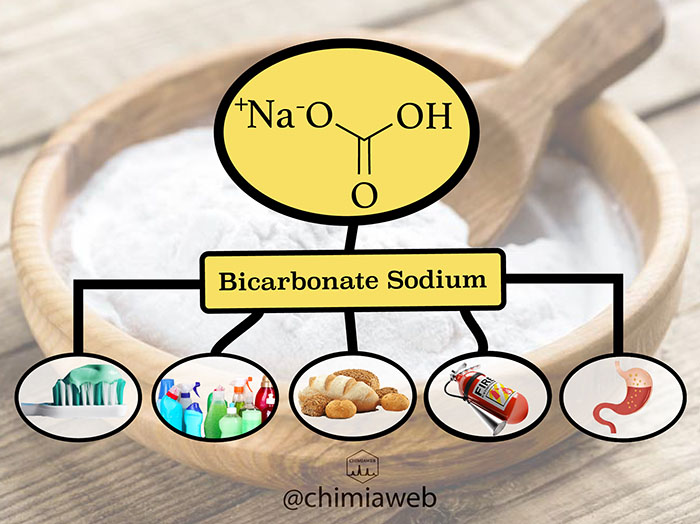
Sodium bicarbonate is a chemical compound with the formula NaHCO3. It is an odorless white powder with a slightly alkaline and bitter taste. It is highly soluble in water and dissolves quickly during mixing. This nontoxic powder is widely available, as it is inexpensive. These useful properties make sodium bicarbonate a multipurpose ingredient in both home use and industrial applications.
Baking Soda
Sodium bicarbonate, commonly known as baking soda, has been widely used in bakery for thousands of years as a leavening agent. When it is mixed with acidic ingredients, a chemical reaction occurs, which releases carbon dioxide (CO2) gas. CO2 gas produces air bubbles that cause the bakery product to inflate and give them a light and fluffy texture.
Abrasive
Sodium bicarbonate has an abrasive nature due to its intrinsic hardness. It has been classified as a substance of low abrasivity. This compound is added to toothpaste formulation to remove dental plaque and stains from dental surfaces. Besides, sodium bicarbonate prevents bacterial growth by keeping the mouth pH basic.
Due to its slight abrasiveness, sodium bicarbonate can also be used in cleaning and detergent products. It effectively removes stains from surfaces without scratching effect.
Antacid
Sodium bicarbonate can act as an antacid. It reacts chemically with gastric acid (Hydrochloric Acid; HCl) to produce CO2 and NaCl, and finally reduce the acidity of the stomach.
Sodium bicarbonate as antacid should be used with caution, as it is absorbed into the general circulation and may disrupt the blood’s acid-base balance. Thus, its use for indigestion relief is limited to a short-term basis to avoid metabolic alkalosis and sodium (Na+) overload.
Fire Extinguisher
Sodium bicarbonate has been one of the important base materials to formulate fire extinguisher powders for years. It is highly efficient, low-priced and nontoxic to the environment. This compound suppresses the flame by producing carbon dioxide (CO2) gas. The released CO2 gas forms a coat over the fire, which drives the oxygen away from burning material. Lack of oxygen causes the fire to put out.