Watermelon is one of the most popular fruits in the world for its delicious tasting and unique aroma. The fresh odor of watermelon makes your mouth water, and it will surely eager you to take a bite of this amazing fruit.
The characteristic cool aroma of watermelon is associated with chemical compounds. These aroma compounds are produced through enzymatic and oxidative reactions. By cutting the fruit, tissue is disrupted and enzymes are released. Exposing to the oxygen, enzymes react with the available fatty acids, especially linoleic and linolenic acids, to generate the aroma compounds.
The identity of the main aroma compounds of watermelon has long been a subject of discussion. It was previously speculated that six- (C6) and nine-carbon (C9) alcohols were the major contributor to the smell of watermelon.
(Z,Z)-3,6-nonadien-1-ol, in particular, was believed to be the most important odor in watermelon because it was relatively high abundant in the isolated extract, and was described as having a high odor detection threshold, which means it can be suitably recognized by the human nose.
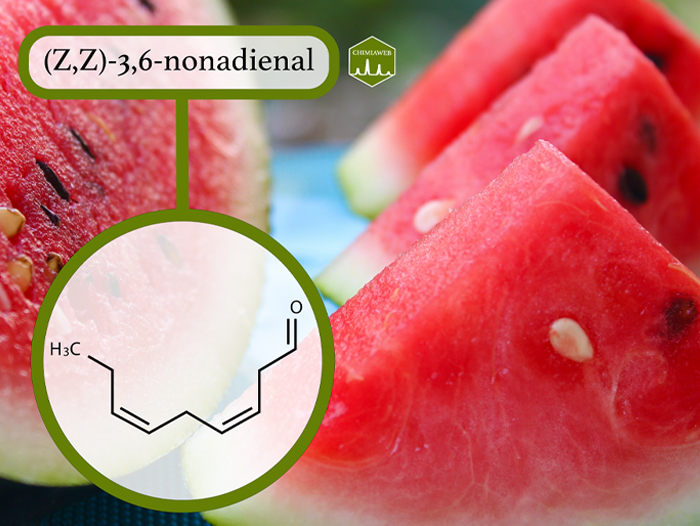
Recent studies showed that C6 and C9 aldehydes are the major compounds responsible for the smell of fresh-cut watermelon. These compounds include (Z,Z)-3,6-nonadienal, (Z)-3-hexenal, (Z)-3-nonenal, (Z)-6-nonenal, (E)-2-nonenal, (Z)-2-nonenal, (E,Z)-2,6-nonadienal and (E,E)-2,4-nonadienal. They are all described to have a kind of “green” smell.
Among the above mentioned compounds, (Z,Z)-3,6-nonadienal is the most predominant aroma compound. It is the only compound that closely matches the characteristic fresh-cut watermelon odor and gives watermelon much of its distinctive aroma and flavour.
(Z,Z)-3,6-nonadienal is an unstable compound in the food matrix and easily decomposes. This makes it difficult to use as artificial flavoring. To develop watermelon-like aroma compounds, several compounds have been synthesized. Although all of them had the fruity odor, none were significantly close to the aroma of (Z,Z)-3,6-nonadienal.
Some people may find the odor of watermelon a lot like cucumber. This comes from the (E,Z)-2,6-nonadienal compound, which is the substance responsible for characteristic odor of cucumber. This particular aldehyde is found in both watermelon and cucumber.