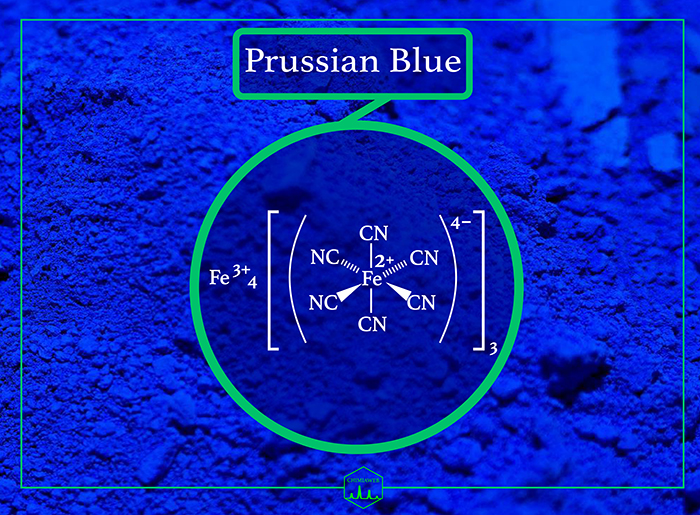
Prussian blue [also known as iron (III) hexacyanoferrate (II)] has long been a link between art and science. Not only did Prussian blue considerably transform the world of paint, but it has also been used for diverse applications.
Despite the fact that Prussian blue consists of cyanide onions in the chemical structure, it is not toxic because the cyanide groups are tightly bound to iron. It is orally used in medicine as an antidote for certain kinds of heavy metal poisoning, such as thallium and radioactive isotopes of cesium. It acts by ion-exchange, adsorption, and mechanical trapping within the crystal structure.